Description
Do you require a test with a Travel Certificate? Click here.
An individual COVID-19 swab kit including RT-qPCR analysis in a leading pharmaceutical and healthcare laboratory (facility is a registered COVID Lab with the Department of Health & Social Care). The test can be taken in the home or workplace without the need for a medical professional and returned to the laboratory via a number of means.
This test can be used for diagnostic purposes, to determine if you have COVID-19 by PCR or for general/workplace screening purposes.
How does the process work?
- Order your test in plenty of time. Place your order before 2.30pm for same day shipping Monday-to-Friday, please note: We operate weekday-only shipping: Orders placed after 2.30pm on Friday will be shipped on the following Monday.
- Register your kit on assuredscreening.com/registration
- Take your sample specimen as per the test instruction leaflet and package for return.
- The customer can choose to return the sample by a means appropriate to their plans. Each kit includes a prepaid Royal Mail TRACKED 24 bag which, through posting in a priority post box, Royal Mail aims to deliver back to us the next working day (see Royal Mail website for further details, their terms of service and service status). Where time is more critical, we suggest returning the sample via one of our drop box locations (no additional cost). In any case, we provide no guarantee and are not liable for any delays or costs due to late return of kits to the laboratory. See further details about returns by visiting our returns page.
- From receiving samples into the laboratory, results will be available typically by the end of the following day after receipt of the test at the processing laboratory via email PDF.
Note, this product is not suitable for Test to Release, Departures or International Arrivals.
Next Generation Test
The laboratory multiplex RT-qPCR kit uses proven primer and probe sequences targeting two different genes from SARS-CoV-2 (assay N1 and assay E) and an internal human control (assay RP). This internal human control ensures that a sufficient quantity of human genetic material has been collected during the sampling process and reduces the risk of false negatives from poorly sampled or unused swabs. The primer and probe sequences have been previously defined by the US Centre for Disease Control (CDC) and the World Health Organisation (WHO) and have been extensively validated for the detection of COVID-19.
- CE-IVD certified test, independently validated by the Department of Health and Social Care
- Test Specificity 99.3%
- Test Sensitivity 98.4%
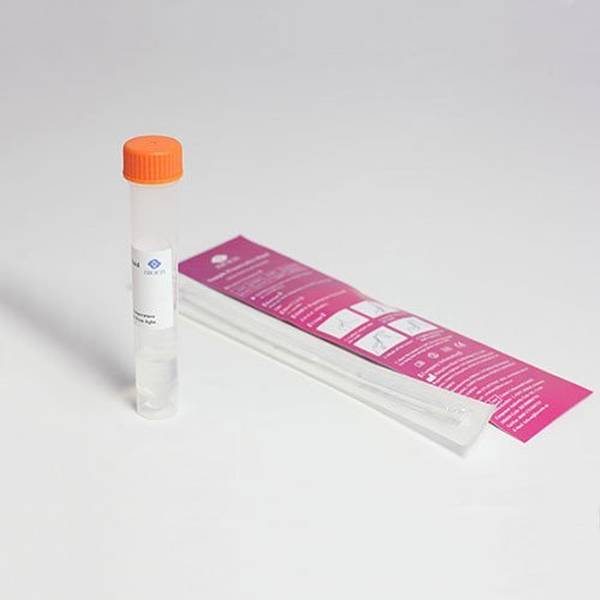
Kit Contents
- CE-IVD Sterile Swab and Specimen tube (containing virus inactivating transport medium).
- Barcode labels with simple online kit registration
- 95 kPa Biohazard transport bag and all return packaging materials in accordance with P650 for UN3373 shipments of category B infectious substances.
- Laboratory testing via CE-IVD approved test carried out by Honeyman Group Ltd, a leading pharmaceutical and healthcare laboratory. Result typically within 24 hours* from receipt of sample at the laboratory.
- Optional return postage via Royal Mail Tracked 24.
- Full instructions.
What is the test kit for?
This test kit provides quick and easy COVID-19 screening of individuals and small groups/businesses.
How does the test work?
The patient is required to provide a swab sample from the back of the throat, tonsils and nose. This swab is placed in inactivating transport media (meaning that should your sample contain COVID, the virus would be inactivated for safe transport).
The laboratory will then carry out the test using a CE-IVD testing kit to determine the outcome of the sample.
Test kits need to be registered individually through our this website prior to use. This means that you can purchase multiple kits in one transaction and use them at a future date (kits have minimum 6 months shelf life) or allocate to multiple people. Results will be returned only to the verified email address provided when registering the test kit.
Product Terms & Conditions
Assured Screening, a trading name of Honeyman Group Ltd provides screening on a best endeavours basis using test kits approved for use in the UK and Europe. Testing provides a historic, momentary result. An individuals health status can change between sampling and reporting of result. All results are based on the sample specimen provided and do not take into account any clinical assessment or patient diagnosis. It is therefore imperative to closely follow sampling instructions and ensure a good quality of specimen collection. Specimens of poor quality may fail processing or provide inconclusive results on test instruments. These will not be refunded. In such cases it is advised that the individual purchase a new test kit for a retest.
*Assured Screening prides itself on speedy sample turnaround and aims to provide these results by the end of the following day after receipt of sample specimen at the laboratory however accepts no liability in the event of a testing delay. All test results and turnarounds are indications and not guarantees. Customers should allow up to 48 hours during peak periods. The laboratory operates 7 days per week, Royal Mail delivers to our lab Monday - Saturday.
All shipping and transport is provided by third parties for which Honeyman Group is not responsible or liable for delay, loss or damage to tests.
This test is designed as a screening test for both symptomatic and asymptomatic individuals and groups. In the event of a positive result and/or displaying symptoms of COVID-19, please follow current government guidelines. Positive results will be passed to PHE for feeding into the NHS track and trace system.
Due to the constantly changing rules and regulations, individuals are responsible to assess the suitability of this test, as well as the returns options against the test requirements for their airline/destination country. Assured Screening is unable to provide any advice or guidance with regards to acceptance criteria for airlines/countries and accepts no liability for this.
THIS PRODUCT IS NOT FOR RESALE. COVID TESTS CAN ONLY BE SUPPLIED BY COMPANIES APPROVED BY THE DEPARTMENT OF HEALTH AND SOCIAL CARE. ANY RESOLD PRODUCTS WITHOUT LICENSE WILL NOT BE TESTED OR REFUNDED.
Returns
If the kit has already been posted to you, you would need to return the unused test kit to us within 28 days of the purchase date so that we could register the test as spoiled. We are able to refund you the laboratory process fee but would need to deduct the cost of the spoiled kit (cost of £25). You would therefore receive a partial refund once we have received the returned kit, together with your order reference number. Once a test has been submitted to the laboratory or returns network, it will be processed upon receipt and is non-refundable.